October 1-2, 2024
The 11th Annual
American Medical Device Summit
Renaissance Schaumburg Convention Center Hotel, Chicago, IL
2024 Featured Speakers

Jennifer Paine
SVP and Chief Quality Officer
Johnson & Johnson
Jackie Kunzler
SVP and Global Head, R&D
Terumo Blood and Cell Technologies
Richard Rapoza, Ph.D.
DVP, Research and Development
Abbott
Jodi Schwartz Klessel, PH. D
VP Engineering/Ultrasound Guidance
Siemens Healthineers
John Sutton
VP, Operations
Thermo Fisher Scientific
Adam Vasquez
Head, Quality, Regulatory and Health Compliance, Google Devices and Services
Google
Pre-Access Registration is closing very soon, on May 17th
About the Med Device Summit
Setting the standard on how the medical device industry connects and exchanges ideas, the American Medical Device Summit provides insights and strategies to enhance the professional development of medtech executives involved in the design, product development, innovation, technology, quality, and regulatory aspects of medical devices
Join the discussion with over 250 of your fellow medical device leaders as we explore the challenges and opportunities in medtech innovation, regulatory harmonization, compliance, digital transformation, and more. At our medical device conference, you’ll hear first-hand case studies presented by our executive speaker faculty with extensive experience driving development and process strategy excellence. Walk away with strategic insights for industry, hospital networks and regulatory bodies to collaborate together to streamline processes, optimize development, design, decrease risk, improve speed to market, reduce costs and remain compliant in a rapidly evolving landscape. This September, we encourage you to join us for two days of thought-provoking content and exceptional networking at our medical device conference designed for executives.
Key Themes
Design
- Drive medical device design and development with a focus on sustaining quality culture and adopting change management techniques
- Enhance medical device industry compliance through proven design controls and effective change management
- Foster medical device design innovation to address evolving healthcare needs and sustain quality gains
- Integrate wearable health tech in medical device design, enhancing patient outcomes and operational efficiencies
Product Development
- Optimize medical device development with advanced prototyping techniques that expedite design validation and market readiness
- Utilize data analysis and evidence-based decision-making to optimize the medical device development process and refine product innovation
- Mitigate regulatory roadblocks to expedite medical device product development
- Ensure compliance in medical device reporting and engineering by adhering to regulatory standards throughout the product lifecycle
Quality & Regulatory
- Navigating the evolving landscape of medical device quality management systems / medical device QMS
- Develop and sustain the gains of medical device quality assurance and adopt proven changes in management techniques
- Leveraging med device compliance and navigating the complexities of medical device regulations
- Identifying the opportunities and challenges in various regulatory markets, alongside an analysis on potential modifications to 510k medical device policies
Innovation
- Revolutionizing healthcare and exploring cutting-edge technologies for medical device innovation
- Discovering the power of medtech innovation, exploring trends and challenges in the ever-evolving landscape
- Exploring cutting-edge and innovative medical device solutions that are optimizing healthcare delivery and improving patient outcomes
- Analyzing the latest medical technology and opportunities for next-gen medical device development
Access The Program
The AMD SUMMIT EXPERIENCE
Executive Corner Podcast
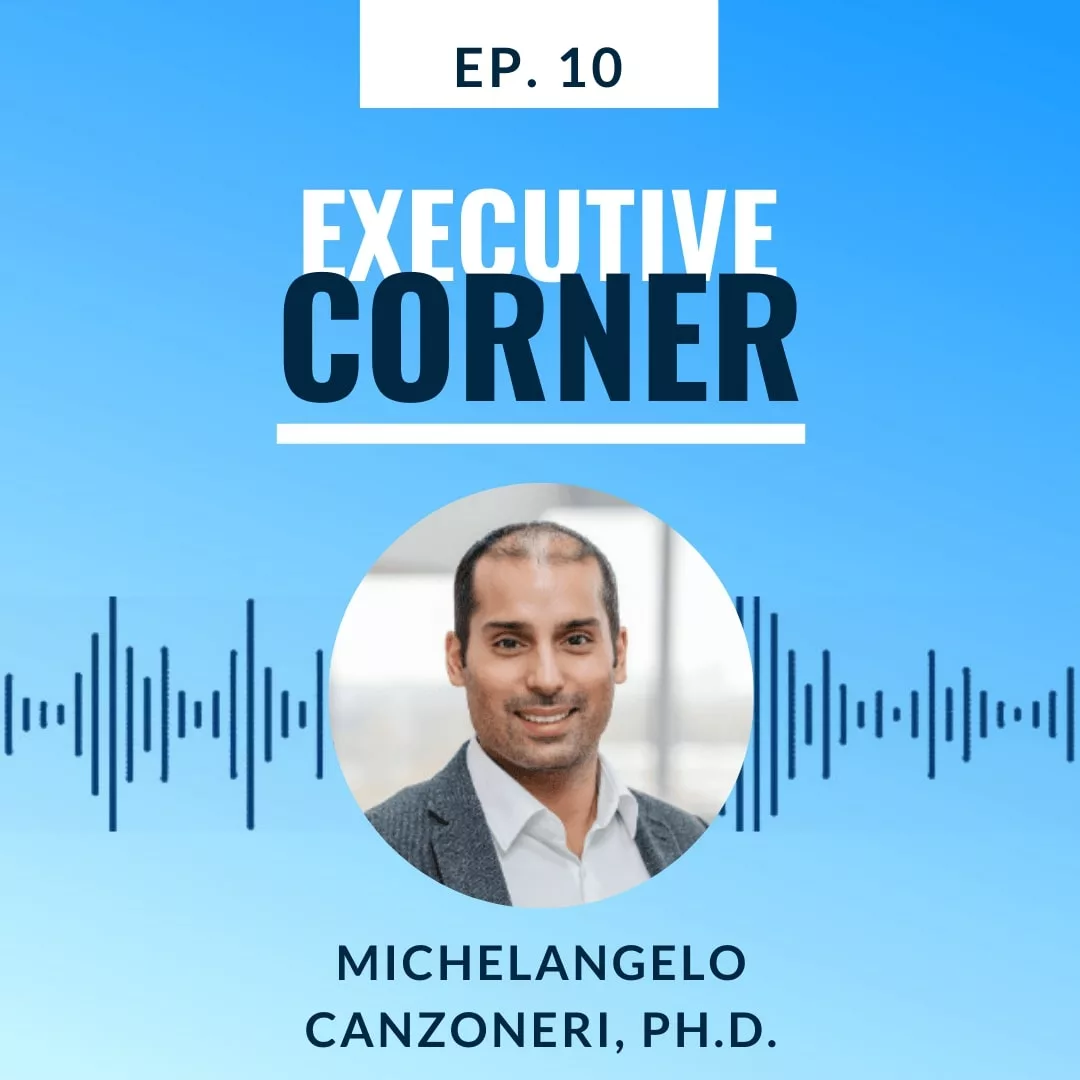
The Power of Curiosity and Why It's Important in Your Transformation Journey with Michelangelo Canzoneri

With Michelangelo Canzoneri, Ph.D.,
Global Head, Digital and Data, Healthcare,
Merck KGaA, Darmstadt, Germany
- How technology is driving data quality, connection, integration and more at Merck KGaA
- What it means to build a digital continuum
- The benefits of data modeling and digital twins
- Why it is important to demystify digital and how to do it in tangible ways
- What to be excited for in the future of healthcare and technology
- The importance of education and being a passionate leader throughout your career
Listen now
Get in Touch
606 – 555 Richmond Street West
P.O. Box 119
Toronto, ON M5V 3B1
Canada
Great conference, relevant and diverse track of topics, lively discussions and excellent networking opportunities.
The conference was very well-organized. The content and shows are relevant and useful. There are ample networking opportunities for all kinds of Medical Device needs!
The Generis folks were friendly and helpful, the vendors were appropriately vetted for the event, and many of the sessions had informative content.
It was an excellent experience getting to understand new developments and innovation within Healthcare and Medical Devices. The Generis team is excellent and on-premise support is amazing.
Great experience, high quality of interactions with industry peers. Well organized and extremely well orchestrated program with agility, by Generis.
Great experience! The 1:1 meetings were high quality and productive.
Event Sponsorship
Engage with targeted medical device industry leaders at the 2024 American Medical Device Summit with Generis’ sponsorship opportunities
Gain direct access to key decision-makers in the medical device industry, from Directors, VPs to Senior Executives as a solution provider. Maximize your ROI with customized packages, extensive networking, and structured 1-to-1 meetings. Explore collaborations in medical device product development, manufacturing, and global regulatory affairs, all while staying abreast of the latest technology and compliance updates. Partner with us for measurable results and drive tangible results!
What Makes Us Different?
Marketing 365®
Our trademarked Marketing 365 initiative allows you to extend your brand beyond the brick-and-mortar medtech event to include content sharing, blog posts, social sharing, original content generation and more - all as a part of your package!
Pre-qualified one-to-one meetings
At our medical device conference, our one-to-one meetings go beyond speed dating, providing you with the opportunity to pre-qualify potential meeting candidates and make adjustments to your meeting schedule before the on-site one-to-one meetings.
Limited Exhibition Floor
We limit the number of sponsors at our medical device event to ensure that quality interactions occur on the floor. We bring only the most relevant, capable and credible medical device executives to our events to meet with our delegates and speaker faculty.
Caliber of Attendee
Generis summits are invitation only. Registrations at our medical device conference are only granted to delegates who meet our strict qualification and seniority standards.
Speaking Opportunities
Unlike other medtech conferences, speaking opportunities at Generis events are tailored to your needs. We offer you direct access to a highly targeted medical device audience that makes the decision on what their businesses will do next.
ONLINE Delegate CATALOG
Full access to specific and detailed business intelligence on each attending delegate including a detailed profile and their key areas of focus/investment via our proprietary attendee database.
To ensure the exclusivity of our medical device conference, we have password protected our pages. Access is granted to qualified individuals and companies who meet the requirements for attendance.
To find out more about Sponsorship Opportunities and the range of services offered in our medical device conference packages, please contact us and we will follow up with you shortly.
Knowledge Center
The Generis American Medical Device Summit brings together innovative, exciting and timely content delivered by today's top minds within the Medical Device industry.
We have developed our medical device knowledge center to ensure that all the past knowledge shared at the events is readily available and accessible to medtech industry practitioners. The knowledge center is an information hub that features insights from our past and upcoming medical device conferences. Gain access to full videos of past presentations, speaker interviews, and a collection of industry insights presented through eBooks and Infographics.
Session Videos

Speaker Interviews
To ensure the exclusivity of our medical device event, we have password protected our pages. Please use the button below to request access to our medtech knowledge center. You will be directed to a login page and provided with the option to request access. If you already have a password, please click on the button below and enter the password when prompted.
infographics

Session videos

eBooks

Venue
Renaissance Schaumburg Convention Center Hotel
1551 Thoreau Dr N, Schaumburg, IL 60173
+1 847-303-4100
Get in Touch
606 – 555 Richmond Street West
P.O. Box 119
Toronto, ON M5V 3B1
Canada